We investigated the the role of the focal adhesion protein Nedd9 on the binding strength to the extra-cellular matrix in mouse embryonic fibroblasts.
Read moreWe investigated to influence of the cytoskeletal linker protein plectin on the mechanical properties of different cell types.
Read moreFilamin A is essential for active cell stiffening but not passive stiffening under external force.
Read moreDesminopathies are the best-studied disease entity within the clinically and genetically heterogeneous group of myofibrillar myopathies, which are morphologically characterized by desmin-positive protein aggregates and myofibrillar changes [1]. The complex molecular pathophysiology of desminopathies seems primarily related to toxic effects of mutant desmin proteins on the formation and maintenance of the extra sarcomeric intermediate filament network. Further, mutant desmin affects essential protein–protein interactions, cell signaling cascades, mitochondrial function, and protein quality control mechanisms [2].
Using primary myoblasts derived from diagnostic muscle biopsies from a patient carrying a heterozygous R350P desmin mutation, we investigated to what extent the expression of mutant desmin contributes to mechanical changes and causes abnormal cellular response to mechanical perturbation. For this purpose, we user our cell stretcher which imposes cyclic mechanical strain to adherent myoblasts on flexible membranes and measured the cell viability in response to cyclic strain [3]. In addition, we performed magnetic tweezer experiments with fibronectin-coated beads to measure cell mechanical properties. Our data provide the first evidence that mutant desmin myoblasts show altered mechanical properties and higher vulnerability to mechanical stretch, which may contribute to the progressive disease process.
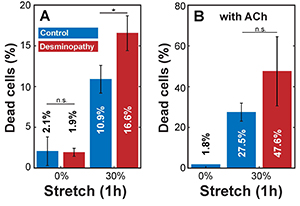
to investigate the response of primary human myoblasts to cyclic mechanical stretch, we cultured the cells on flexible PDMS substrates and exposed them to uniaxial cyclic stretch with a peak-to-peak amplitude of 30% at 0.25 Hz for 60 min. In timematched control experiments (no stretch), the percentage of dead cells was around 2% in both control and diseased cells (Figure 1A). The percentage of dead and detached cells after 1 h of cyclic 30% stretch was 10.9% in control cells and 16.6% in mutant desmin cells.
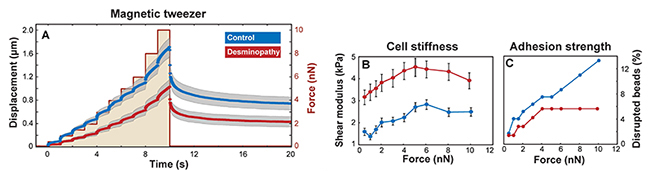
We measured the stiffness of single myoblasts using magnetic beads that were attached to integrin cell surface receptors and laterally pulled by a magnetic tweezer device. Beads on mutant desmin cells moved significantly (p < 0.05) less in response to lateral force compared to control. From the bead displacements (Figure 2A), we computed the shear modulus or stiffness of the cells. Our data show that human mutant desmin cells are approximately 2 times stiffer compared to control, regardless of the applied force magnitude (Figure 2B). The higher stiffness indicates that mutant desmin cells have a higher baseline prestress [4,5]. This interpretation is further supported by the observation that mutant desmin cells showed a 30% increase in stiffness with increasing force, whereas controls stiffened by nearly 100%. Since the total cytoskeletal prestress is the sum of baseline prestress and externally imposed stress from the magnetically forced beads [6], cells with a higher baseline tension (desmin mutant cells) respond to increasing external forces with a smaller relative increase in stiffness (Figure 2B).
According to Hooke’s law, mechanical stress increases in direct proportion with stiffness and stretch amplitude. We reasoned that the higher number of detached cells and cell death after stretch in mutant desmin cells may be due to higher stretch-induced cell stress arising from higher stiffness. To test this hypothesis, we stimulated actomyosin contraction in cultured myoblasts by adding 0.6 mM Acetylcholine (ACh). Since cell stiffness increases linearly with cytoskeletal prestress [5], we expected a higher fraction of dead or detached cells after ACh treatment in response to stretch. Indeed, we found in ACh-activated controls a high percentage (27.5%) of dead or detached cells after cyclic stretch, which is a 2.5-fold increase over stretched, but non-ACh-treated cells (Figure 1A and B). In ACh-treated mutant desmin cells, the percentage of dead or detached cells after stretch increased to 47.6%, which is about a 3-fold increase over the stretched, but non-ACh-treated cells. Experiments with ACh-treated, non-stretched cells showed no significant increase in the number of dead or detached cells compared to non-stretched and non-treated controls (Figure 1A and B).
To rule out that the increased number of detached mutant desmin cells during stretch was the result of poor matrix adhesion, we applied an increasing lateral force protocol to integrin-coupled magnetic beads. This approach allowed us to record the force at which the beads detach from the cells and to estimate the adhesion strength. The fraction of beads that detached from mutant desmin cells was lower than in control cells at all force levels (Figure 2C), indicating that mutant desmin cells show a high adhesiveness to the extracellular matrix. We conclude that the higher number of detached mutant desmin cells was due to an increased number of cell death rather than poorer matrix adhesion properties.